Memory Springs
Memory Springs
If you haven't already seen our video of a shape memory alloy, then check it out below:
Please allow social and marketing cookies to show embedded content.
A fantastic example of an amazing property of some alloys that is caused and controlled by the arrangement of the atoms - the ability of a solid piece of metal to ‘remember’ a previous shape! These Shape Memory alloys, made from titanium and nickel, can be used to control and optimise airflow in jet engines where conventional hydraulic or electrical control systems would be unable to operate. But how do they work?
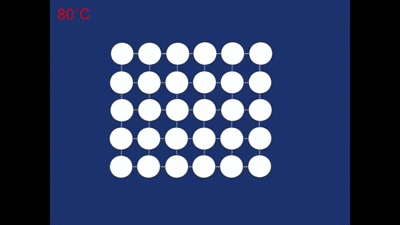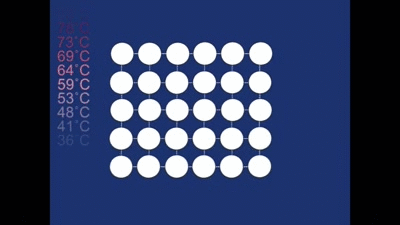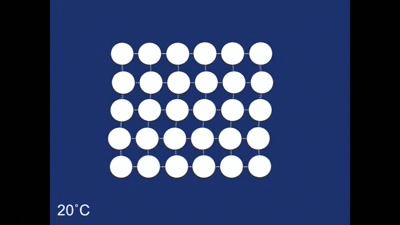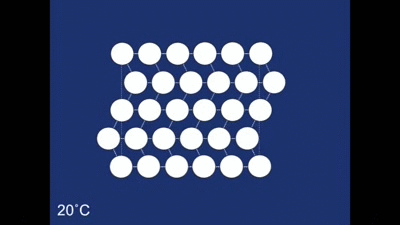
The Shape memory effect is controlled by a structural rearrangement on the atomic scale. At room temperature the atoms from the structure shown below:
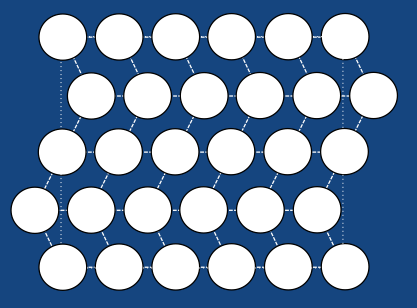
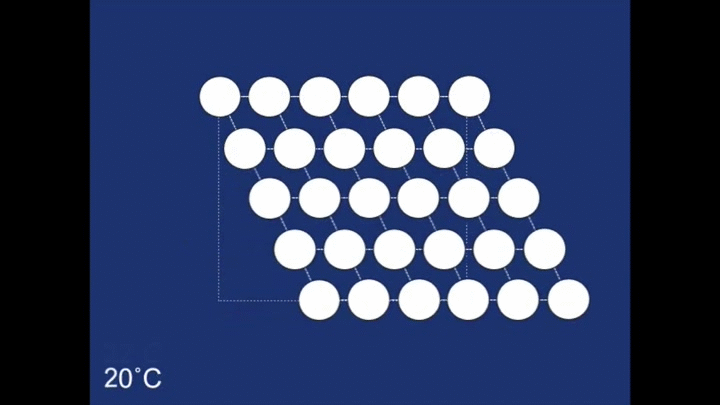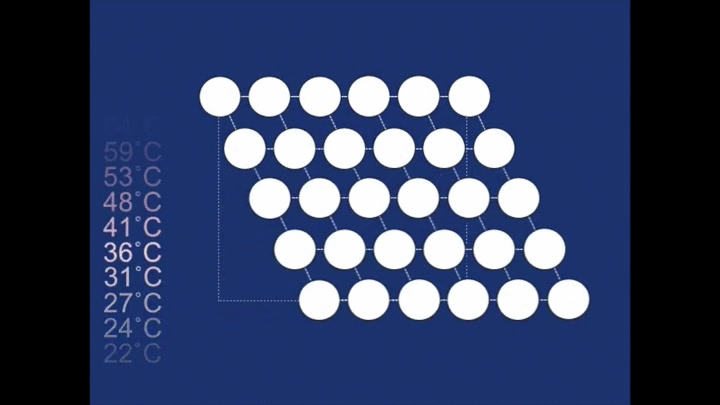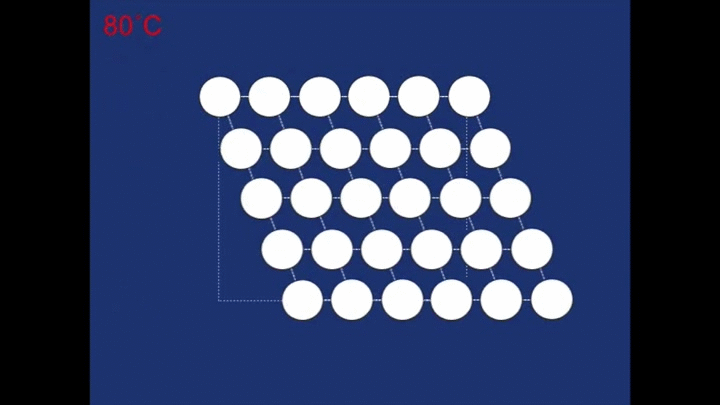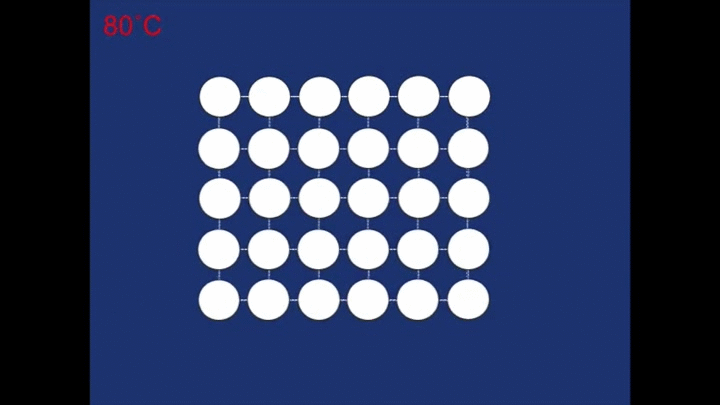
The structure as you can see is made up of many rhombehedra - but some of the different layers are pointing in opposite directions. The layers therefore are mirror images of one another. The structure is therefore said to be twinned. In this case the twinning forms naturally on cooling, but it in other systems it can occur in other ways (find out more about deformation twinning in calcite). The atomic structure of the spring looks like this when the spring is in its coiled-up state at room temperature. When we then stretch the spring out we remove this twinning. We can see what is happening to the structure as we do this:
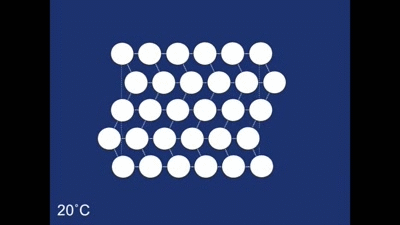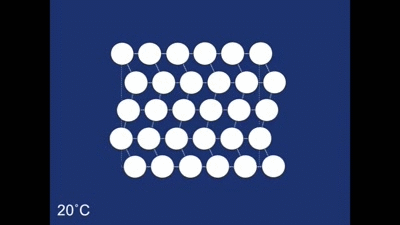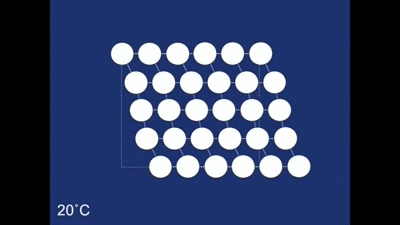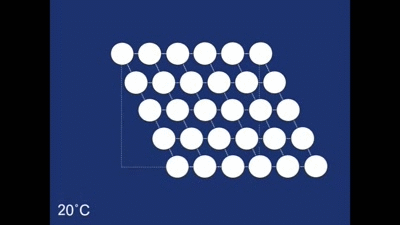
The final structure of our deformed piece of metal is the non-twinned structure (all the rhombehedra are pointing in the same direction). So what happens when we heat it in the boiling water? The change to the structure can be seen below:
At the high temperature the structure relaxes back to a different structure - a cubic structure (the atoms are arranged at the corners of cubes). It is this change in the structure that is responsible for the spring 'remembering' its previous shape.
As it cools down naturally the cubic structure changes into the twinned structure again. This is easy for it to do as the overall shape of the crystal doesn't need to change very much (notice how the motion is only small compared with the motion that occurred during deformation)! It has to twin, rather than all the rhombohedra forming in the same orientation, since it needs to retain the same overall shape.
The alloy can then repeat this cycle over and over again - being deformed to the non-twinned structure, heated into the cubic structure and then cooled back into the twinned structure.
