Tempering Steel
Please allow social and marketing cookies to show embedded content.
What is going on?
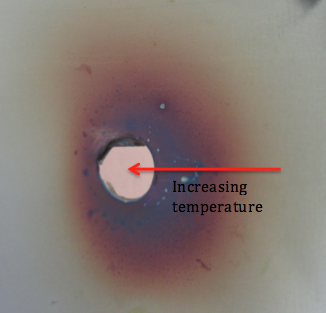
In the video we can see a thin stainless steel foil being heated by a candle. You should notice that as the steel is heated, the colour changes, resulting in the coloured surface seen in the picture.
Steel is a classical example of a type of material called an alloy, made by dissolving elements in one another. Steels are made by mixing a small amount of carbon into iron. This process, known as alloying, can radically change the properties of the material, making them harder or stronger or environmentally resistant.
As the steel is heated, oxygen in the air reacts with the steel surface forming compounds called oxides. Stainless steels, like this one, also contain the element Chromium. It is the Chromium oxide that forms during the heating that gives rise to the colours we see. Depending on the thickness of the oxide layer, we see a different colour. This thickness then depends on the temperature reached by the material at that point. We can therefore use this colour to suggest how the temperature varied across the surface during the heating process (shown on the diagram). The colours and their related temperatures are as follows: Golden (200-240°C), Red (240-270°C), Violet (280°C), Blue (290°C), Grey (over 360°C).
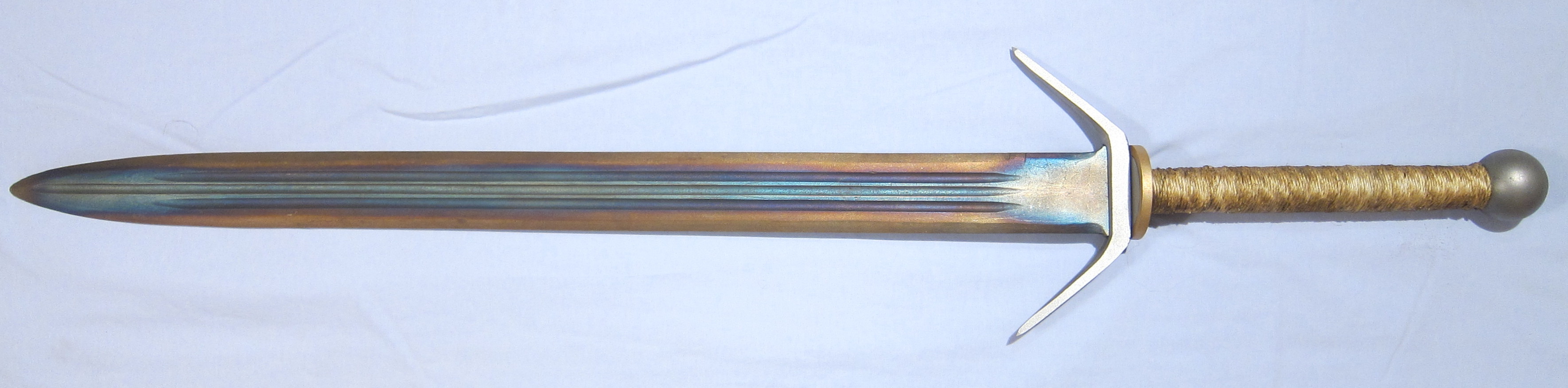
Blacksmiths use these colours to indicate the temperature to which the alloy has been heated to, and therefore the physical properties it may have. The picture below shows a sword that has been heated in this way – a process called tempering. The golden colour on the edge of the blade shows that the metal was much cooler here compared to the middle. This makes the edge of the blade very hard, but the centre of the blade remains softer. This is essential for swords and knives as it provides the toughness needed for the edge of the blade but prevents the blade from becoming very brittle – which would lead to the sword shattering when you tried to use it!