Deforming Calcite
/p>
What is going on?
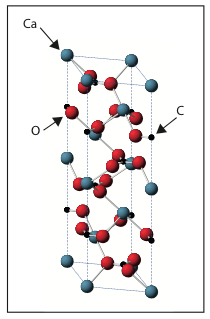 Calcite is a mineral made of carbon (chemical symbol C), calcium (Ca) and oxygen (O). For every carbon atom there is one calcium atom and three oxygen atoms. Calcite is a crystal (find out more here) and therefore is made up of the same building block repeated over and over again. A picture of this diamond shaped building block is shown below.
Calcite is a mineral made of carbon (chemical symbol C), calcium (Ca) and oxygen (O). For every carbon atom there is one calcium atom and three oxygen atoms. Calcite is a crystal (find out more here) and therefore is made up of the same building block repeated over and over again. A picture of this diamond shaped building block is shown below.
The consequences of this regular repeating structure can be seen macroscopically; first in the shape of the overall crystal, and secondly when the crystal breaks. Calcite always breaks along certain direction, which correspond to specific layers of atoms. This process is called cleavage, and is the same mechanism that enables thin slate roofing tiles to be produced. Imagine you have a tower of Lego bricks, if bent, it will always break at the joins between the bricks. The same idea applies to the calcite crystal. When it was hit with the hammer in the video, it broke along its weakest directions, producing smaller regular shapes, which reflect the underlying crystal structure.
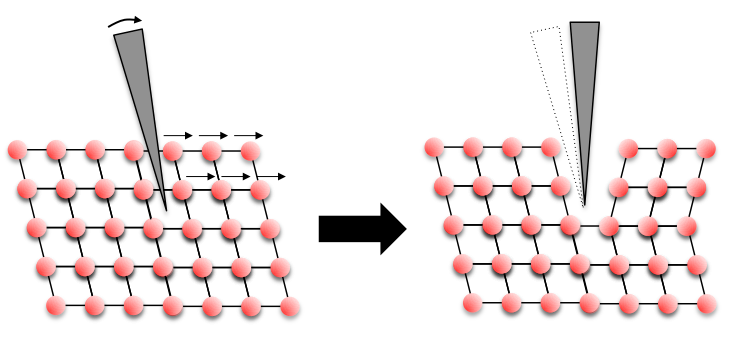
You will also have seen the Calcite crystals being deformed more carefully with a knife. Here we are seeing a slightly different process called deformation twinning. A schematic of the process occurring is shown below.
![Calcite2 Schematic of Baumhauer's method [1] of twinning deformation that occurs in Calcite, adapted from Johnsen [2]](https://www.eng-atoms.msm.cam.ac.uk/files/styles/large/public/media/Calcite2.png?itok=jXz3Z1I2)
As the knife blade is forced in between the layers of atoms it pushes or shears the layers over to one side. The layers atoms, forced by the knife, slide into a position that is the same as the original structure, but just reflected. This produces the twinned crystal - a mirror image of the original crystal!
For us, as materials scientists, it is incredibly important to understand how materials deform when they are put under different types stress (simply hit roughly with a hammer, or carefully pushed with a knife blade). If we can understand how they deform, we can then start to work out how to prevent this deformation and so create new materials with more desirable properties!
S. J. Lainé, R. Muñoz-Moreno & L.R. Owen
1. H. Baumhauer, "Ueber künstliche Kalkspath-Zwillinge nach – ½ R," Zeitschrift für Krystallographie,3, 588 (1879).
2. A. Johnsen, "Schiebungen und Translationen in Kristallen," Jahrbuch der Radioaktivität und Elektronik, 11, 226 (1914).
