What is an Atom?
What is an Atom?
 Richard Feynman (Nobel Prize winner for Physics) once asked the following question:
Richard Feynman (Nobel Prize winner for Physics) once asked the following question:
“If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words?”
His answer: ‘The atomic hypothesis - that all things are made of atoms’
Everything in the universe is made up of tiny building blocks called atoms. Atoms are incredibly small. If you were to line up atoms along the edge of a sheet of paper, there would be around a billion (1,000,000,000) of them! In a glass of water there are around 1,000,000,000,000,000,000,000,000 atoms – that is more than the number of stars in the universe!
If you think about solids, liquids and gases:
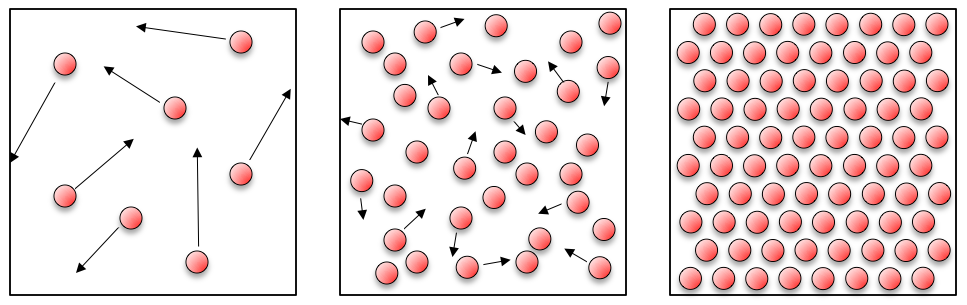
In a gas the atoms are quite spaced out and have lots of energy, and bounce around all over the place. It is this energy that means that gases expand to fill containers (like the room you’re sitting in!). In a liquid the atoms have less energy, but still move around bumping into each other. This arrangement of atoms means that liquids are able to flow and fill the bottom of containers. For a solid, the atoms are fixed in place – they may have a little bit of thermal energy causing them to shake or shiver, but on the whole they don’t move.
The arrangement of the atoms and the structures they form is therefore incredibly important to the properties of the material. We can change these structures by changing the atom type (i.e. changing the atom size and electronic properties). Different types of atoms are called elements, some of which are quite familiar – Oxygen, Carbon, Nitrogen, Iron, Gold. Chemists are interested in understanding how the different elements interact with one another and often use the periodic table (see Fig), which lists all the known elements:

Or if you fancy you can hear the elements sung:
For metallurgists, the periodic table is our playground. We can take different elements and by mixing them together, in a process called alloying (find out more here!), create new materials with improved properties.
